The Amine Unit
Amine Unit Introduction and Process Flow
An amine unit is a “Utility” type unit. Without it, a refinery would have to shut down. It circulates lean amine through the plant, supplying absorbers with amine to absorb H2S from its gas feeds, cleaning those gas feeds up to route them on to other units for processing, or to supply the Refinery Fuel Header.
Rich amine from the absorbers is then routed back to the amine unit to be regenerated and used again as lean amine, and then routed back to the refinery absorbers. Rich amine flows to a common header back to the Amine Hydrocarbon Flash Drum. It is then routed to feed exchangers that heat the Amine before entering the Regenerator. The Regenerator has a reboiler that supplies the necessary heat to strip H2S and CO2 from the rich amine, using steam as the heat medium. As released gases rise and exit the tower, they are routed to overhead condensers lowering overhead temperatures. Condensed liquid settles in a reflux drum. Liquid from the reflux drum is pumped back to the Regenerator to control overhead temperatures. The clean or acid gas from the overhead is routed to the Sulfur plant for processing.
The regenerator bottoms, now considered lean amine, is routed through the feed/bottoms exchangers for cooling and to the Lean Amine tank, to once again start the journey to be circulated to the Refinery Absorbers.
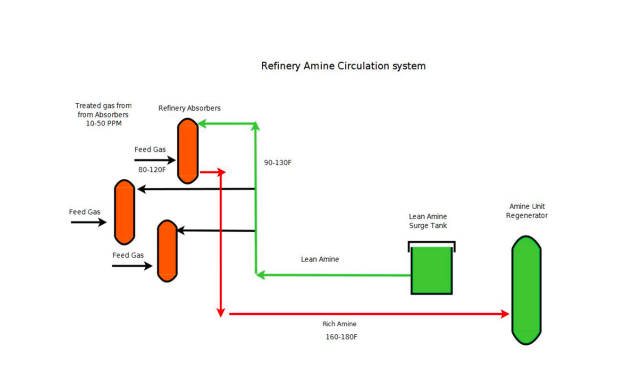
Refinery Amine Circulation System
Basic Chemistry of Amine
The Refiner has a wide variety of Amines to choose from. They need to determine how much and to what concentration it will remove H2S. Costs also need to be evaluated. Prior to using Amine, caustic was commonly used. Unfortunately caustic is costly as it cannot be regenerated. Concerned Refiners should be aware when choosing an Amine to include the following: The sweetened gas meets the required purity specifications with respect to H2S and C02. Selection should take into account the present refinery equipment and move toward minimizing plant operating costs. The following questions should be considered during evaluation:
- Can the Amine circulation rate be reduced by selecting an amine which may be used at a higher concentration and/or solution acid gas loading?
- Can the reboiler/condenser size and duty be minimized by use of an amine which requires a lower circulation rate, and/or which has better heat efficiency with H2S and C02?
- Can the absorption of H2S and CO2 be optimized by use of an amine mixture?
- If there are corrosion problems, is there an amine or mixture of amines more resistant to degradation?
- Could an alternative flow scheme increase the efficiency of the process?
Alkanolamines are bases used in Refineries to remove H2S, Mercaptans and CO2 contaminants. They are divided into three different types: Primary, Secondary and Tertiary, depending on whether one, two or three hydrogen atoms have been replaced by an organic group:
- Monoethanlamine (MEA) Primary
- Diglycolamine (DGA) Primary
- Diethanolamine (DEA) Secondary
- Diisopropylamine (DIPA) Secondary
- Methyldiethanolamine (MDEA) Tertiary
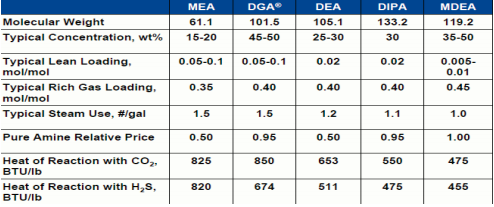
Commonly used Amines and their Properties
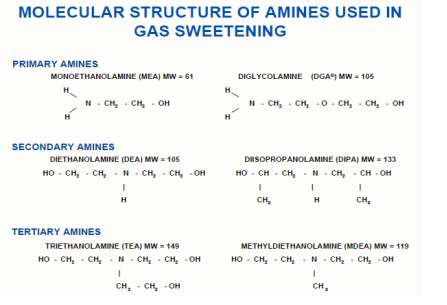
Molecular structure of amines used in gas sweetening
Let’s look at the advantages and disadvantages of selected Amines.
MEA: Typical concentration weight 15-18%. PROS – It is low cost and the degradation products can be removed by reclaiming CONS – CO2, COS and CS2 can irreversibly degrade the Amine. The degradation products are very corrosive. Degraded Amine has less ability to remove Acid gas. High energy consumption is needed for its regeneration.
DGA: Typical concentration weight 50%. PROS – It can achieve low specifications and degradation products can be reclaimed. CONS – High Cost, CO2, COS and CS2 can irreversibly degrade the Amine. Degradation products are very corrosive and are highly soluble in aromatic, olefins and heavy hydrocarbons. High energy consumption is needed for its regeneration.
DEA: Typical concentration weight 25-35%. PROS – Moderate cost, degrades less with CO2 and COS than MEA. Less energy is required for its regeneration than MEA. PROS – Not selective in the presence of CO2. CO2 and COS can irreversibly degrade the Amine. Degraded Amine has less ability to absorb acid gas.
DIPA: Typical concentration weight 27%. PROS – Some selectivity in the presences of CO2, moderate energy requirements for amine regeneration. CONS – High cost, CO2 and COS can irreversibly degrade the Amine.
MDEA: Typical Concentration weight 35-50%. PROS – High selectivity for removing H2S, higher acid gas removal capacity at the concentration of use, does not degrade with CO2 or COS, little or no corrosive issues at concentration of use. Low energy required for Amine regeneration. CONS – Higher cost than MEA, DEA and DIP, more soluble than DEA in liquid hydrocarbons. MDEA is becoming a more popular Amine because of the advantages it offers. Because it is a tertiary Amine, the MDEA molecule provides a significant improvement to acid gas capacity per gallon on of solution. It reduces the risk of corrosion, energy needs and it allows CO2 to slip through the absorbers and not increase regeneration.
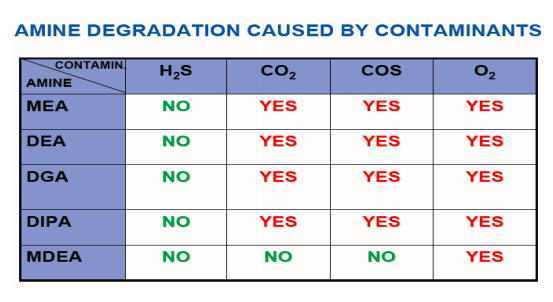
Amine degradation caused by contaminants
Weighing all the options a Refiner might consider for choosing an Amine, there is still another part of the equation to consider in an Amine unit – Heat Stable Salts (HSS). What is that, you ask? Organic and inorganic salts formed by Amine reacting with Oxygen create a reaction between the Amine and organic or inorganic acids. They are acid anions and have a stronger acid strength than the acid gas and bind to the Amine. Once Heat stable salts are formed they cannot be regenerated out of the tower. Nor can they be removed by use of mechanical or carbon based filters. The impact of HSS reduces the strength of the Amine, causes foaming and can cause corrosion especially in areas where there is high temperatures and low pressures, such as the reboiler.
Typical HSS include acetate, formate sulfate thiocyanate, and thiosulfate salts. The HSS accumulate over time in a circulating system and will reduce the scrubbing capacity. If the HSS concentration exceeds a critical level, they will need to be removed or neutralized.
Stay tuned for Installment IV of our Sulfur Recovery Series, where we continue exploring the Amine System. For more information about sulfur recovery and to learn about our consulting services, visit our Field Services Consulting page.







Leave a Reply
You must be logged in to post a comment.