We are back with the second installment in our Sulfur Recovery 101 Series!
Properties of Hydrogen Sulfide: The devil’s in the details.
Hydrogen sulfide has been studied for hundreds of years. In the 19th century, Dutch pharmacist Petrus Johannes Kipp invented a device that would generate a variety of gases using liquids and solids as regents. He called it the Kipp Generator (no surprise there). Today it would be quite foreign to lab chemists. H2S and H2O both contain hydrogen, but that’s where their commonalities end. H2O contains oxygen and is harmless. H2S contains sulfur and can kill you.
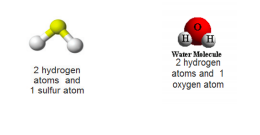
Hydrogen sulfide is a colorless gas with a distinct, offensive odor at low concentrations and is said to smell like rotten eggs. It is heavier than air and is flammable. H2S is somewhat soluble in water and it reacts with strong oxidizers, nitric acid and metals.
Simply put, if you have process with H2S running through the pipes and towers, you’re going to have to pay attention to corrosion issues. One issue refiners struggle with is the “Wet H2S cracking” problem. It occurs in different concentrations, dependent on the unit process and wherever there is a wet H2S environment, such as fractionator’s overhead drums, absorber and stripper towers, and separators.
There is a wide range of damage mechanisms that can occur due to the effect of hydrogen charging in wet H2S environments. One of the more common problems is SSC– sulfide stress cracking. The H2S causes the hydrogen to enter the metal structure rather than bubble off from the surface. To protect from SSC, HIC (hydrogen induced cracking), and other damage mechanisms, refiners use a variety of defenses. They include chemical injection, such as Ammonium Polysulfide, upgraded metal cladding, and coating. Hydrogen sulfide can cause a buildup of iron sulfides (FeS). If iron sulfides are exposed to oxygen and dry climate, it can lead to a pyrophoric situation, igniting in less time than it takes you to look up the word “pyrophoric.” When burned, H2S will create S02, sulfur dioxide.
Safe Handling of H2S 
We all have heard how hydrogen sulfide smells like rotten eggs. But remember, that is in small concentrations. If it is in a high enough concentration, it will simply kill you. Since H2S is heavier than air and may travel along the ground, it will collect in low-lying and enclosed, poorly-ventilated areas such as manholes, vaults and sewer lines. The primary route of exposure is inhalation; the gas is rapidly absorbed by the lungs. In high enough concentrations, the ability to smell the gas can be lost instantaneously. Therefore, DO NOT RELY on your sense of smell to indicate the presence of H2S. In addition, hydrogen sulfide is highly flammable and gas/air mixtures can be explosive. If ignited the gas burns to produce toxic gases. Below are solid guidelines to follow when dealing with H2S Systems:
- Engineer and design systems that keep exposure at a minimum.
- Areas containing H2S should have adequate gas detector monitors, egress pathways and evacuation procedures in place.
- Personnel should have personal H2S monitors, respirators and SCBA readily available.
- Never enter an area containing hydrogen sulfide without proper training and authorization.
H2S Exposure ranges
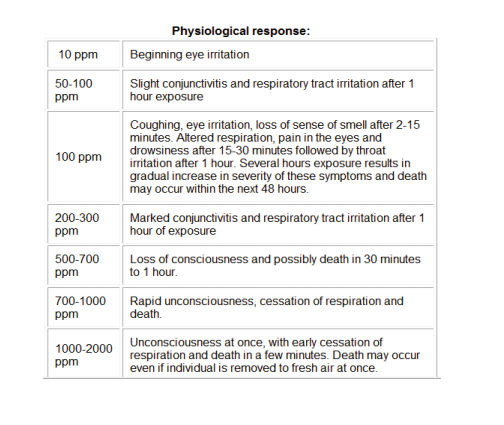
H2S Exposure Ranges
Stay tuned for our next exciting installment, where we reveal the mysteries of Amine and the Amine Unit.







Leave a Reply
You must be logged in to post a comment.