(This is part of an installment series on Sulfur Recovery)
Most of us are familiar with sulfur. We know it is a key ingredient used in gunpowder, matches, and fertilizers. And if you’re in the refining business, you know it’s a necessary component to processing oil. But there’s a lot about sulfur you may not know. Does the oil and gas industry profit from processing sulfur? Why is the EPA concerned about “low sulfur” and “ultra low” diesel? And what do you really need to know about SO2 emissions? This multi-part series will answer those questions and many more as it explores every facet of sulfur recovery. So let’s get started!
What is sulfur and where does it come from?
Sulfur is a naturally occurring element found in many areas throughout the world. It is especially prevalent in volcanic regions. It can be found as a pure element and as sulfide and sulfate minerals. It is an essential element for life found in two amino acids: cysteine and methionine.
The oil and gas industry is concerned with fossil-based sulfur, which helps to produce the world’s energy needs. In relation to sulfur, crude can be broken down into two groups: sweet and sour. Sweet crude contains less than 0.5% sulfur, compared to sour varieties, which contain a considerable amount more sulfur. Sweet crude also contains smaller amounts of hydrogen sulfide and CO2. Sour crudes tend to be more corrosive and require the removal of more impurities before they can be refined into energy products like gasoline and low sulfur diesel. Crude prices vary, but sour crude is typically cheaper than its sweet sibling. And because of its impurities and corrosiveness, refiners need to ask themselves whether or not the lower price will offset the costs of equipment use and abuse from processing sour.
H2S – Hydrogen Sulfide from cradle to grave
Hydrogen Sulfide is present in numerous crude waste water and gas streams within the refinery. These streams usually contain carbon dioxide, water vapor; trace quantities of hydrocarbon and sulfur. Gases containing ammonia are considered sour or “dirty” gas. While gases without ammonia are considered acid gas or “clean” gas, sulfur must be recovered from these streams. The gas streams originate in various units and are processed through an absorber. But the water condensed in overhead systems is considered sour and is very acidic and corrosive. Gas containing H2S will be routed through an absorber. Sour water containing H2S will be routed to a sour water stripper.
The H2S absorber and those nasty gases
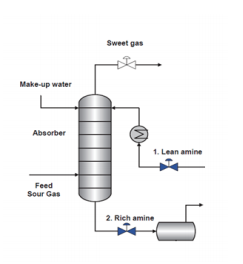
Refinery absorbers are also known as scrubbers or contactors. Their purpose is to “scrub” out the hydrogen sulfide or, in other terms, absorb the H2S through the Amine. These terms are interchangeable.
Lean Amine (clean, regenerated amine) flows counter-currently to the sour (H2S-laden) gas in the absorber. Entering at the top section of the absorber, the lean amine flows downward, across tower trays, coming in contact with the rising sour gas stream. The Amine absorbs H2S; the acid gas dissolves in water and reacts with the amine to form a salt that holds it in the amine solution. The equilibrium reactions that take place in the absorber is represented by the following:
H2S (gas) ↔ H+ + HS ̄ (in solution)
Hydrogen Sulfide shares a reversible reaction with excess hydrogen ions (meaning its low pH or acidic with the Hydrogen Sulfide Ion (slightly alkaline) amine solution. As the reaction takes place, more OH ̄ (Hydroxide Ions) is produced, increasing the pH. The higher pH ionizes more H2S, as it is dissolved in the amine solution and the better the acid gas is scrubbed.
- Amine is alkaline
- H2S is acidic
- Reversible acid-based reaction.
Amine
Once Amine is enriched with H2S, it is known as Rich Amine. The process conditions that favor absorption of H2S are high pressure and low temperature. Absorbers are controlled with tower temperatures usually running between 90 and 120 °F. Individual refinery units will adjust temperature controls depending on what the sour gas feed temperature is running.
The pressure of the particular Absorber is also dependent on that unit’s process. As an example, it can run anywhere from approximately 60 to 850 Psig. The Lean Amine routed to refinery absorbers typically runs 10 to 15 ̊F warmer than the sour gas entering the absorber. The goal is to have it warm enough to absorb the H2S and to keep from condensing hydrocarbons and cause the absorber to foam.
What is foaming, you ask? It can be caused be several things. Here are just a few:
- Dirty Amine
- Feed Composition
- Condition of the Absorber (trays/packing dirty or clean
- Dp (differential pressure) of the Absorber
- Process variables (temperature/Pressure)
From the Absorber, Rich Amine is routed to an Amine run, where it is regenerated back into Lean amine. This will be covered further in the Amine Unit Section.
Sour water
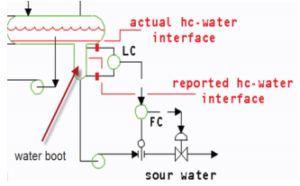
Sour Water: Where it comes from and where it goes
As previously mentioned, crude oil contains organic sulfur and nitrogen compounds. The refining processes convert the compounds into H2S and some nitrogen compounds to ammonia NH3. Steam and water that are used in most processing eventually condense out and collect in process low points, such as water boots.
Both H2S and NH3 are water soluble and therefore produce contaminated “sour” water. Sour water may contain other contaminants as well, such a carbon dioxide, phenols, cyanide, organic and mineral acids. Again, crude makeup will determine contaminants and their quantities. From various refining units, sour water is typically routed to a sour water unit. Although there are several methods of treating sour water, steam stripping is the most common and the simplest method for removing contaminants.
Claus unit: the marriage between amine and sour water

Claus unit
The Claus process is probably the most significant gas desulfurization technology for recovering elemental sulfur from hydrogen sulfide. First patented in 1883 by the scientist Carl Friedrich Claus, it is the most common process used in the industry. The Claus technology can be divided into two process steps: thermal and catalytic. To obtain the end product the unit feed consists of H2S gas, also known as clean gas or acid gas, routed from the refinery amine plant. Sour water gas, or “dirty” gas, which is made up of H2S, NH3 and H2O is routed from the sour water stripping unit overhead to the front end of the sulfur unit for further processing.
Tail Gas: The end of the road
There are several technologies available for treating tail gas. But the purpose remains the same. The unit is designed to substantially recover the remaining sulfur compounds from the Sulfur Plant Effluent gases to satisfy government emission control standards. Tail gas recovery consists of chemically converting sulfur compounds to H2S, treating the H2S and routing it to an incinerator.
Stay tuned for the next installment in our series, which discusses the properties of hydrogen sulfide and procedures for safe handling of H2S.
3 responses to “Sulfur Recovery: The Big Picture”
Leave a Reply
You must be logged in to post a comment.







I’ve a very basic doubt regarding the Claus reactions. The following are the two reactions in Claus furnace:
1) H2S + 3/2 O2 —> SO2 + H2O
2) 2H2S + SO2 —> 3/2 S2 + 2H2O
I have always been confused if the 2nd reaction is exothermic or endothermic? Any clarifications will be appreciated. Thank you.
Hello heisenberg93,
The H2S and SO2 reaction in the thermal stage (reaction furnace) is at the high-temperature zone. It produces S2 and endothermic in nature.
In the catalytic stage, the reaction produces S6 and S8 at a low temperature and exothermic in nature. The exothermic reaction can be observed by having a higher bottom bed temperature compared to the converter inlet temperature.
I hope this helps.
[…] Sulfur Recovery: The Big Picture […]